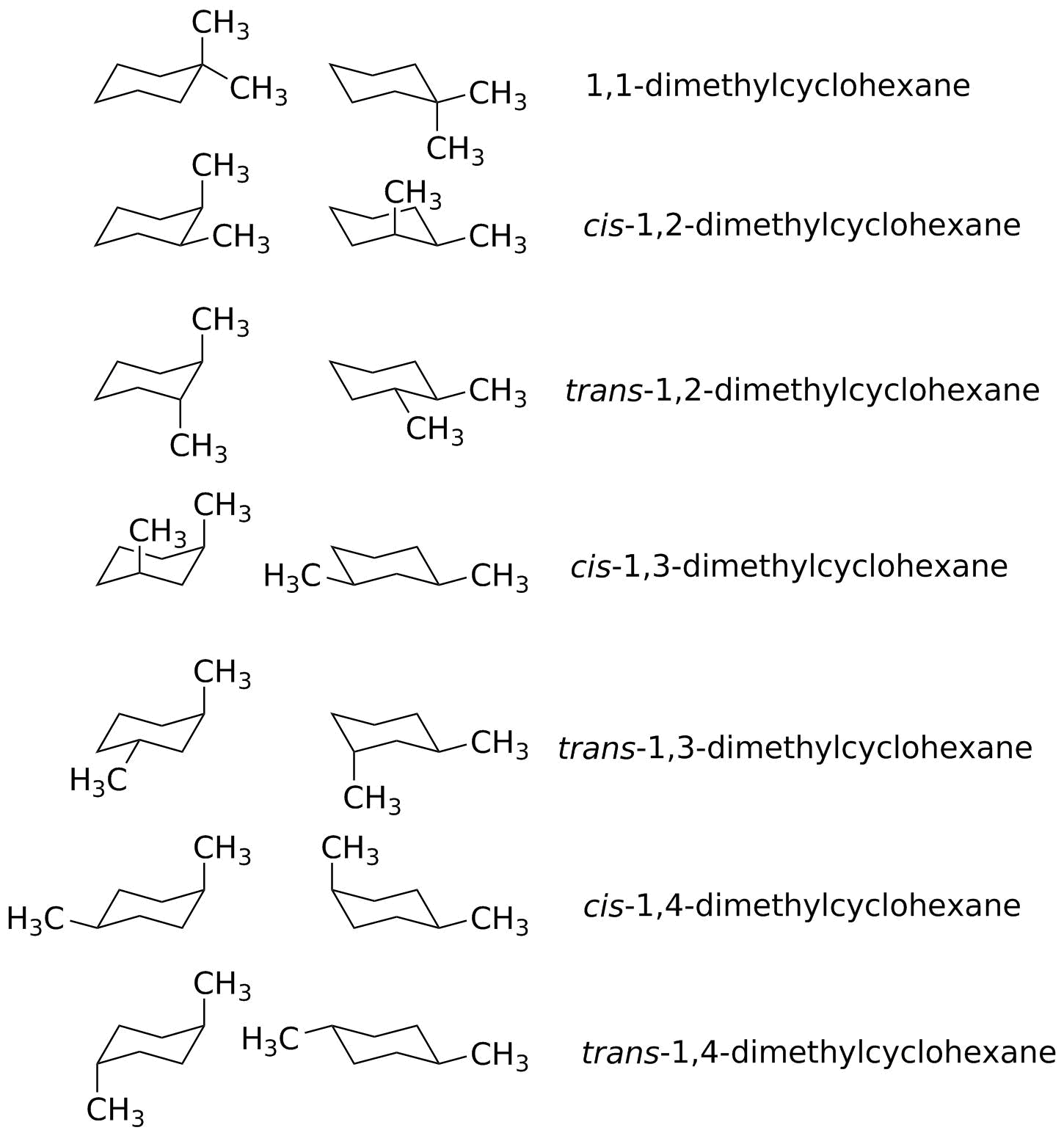Barber chair drawing conformations cis and trans from haworth diagrams black white design artists steel autocad. This rehab they ch three ch three.

Cyclohexane Chair Conformation With Cis And Trans Youtube
Thats not how you decide it at all.
Chair conformations cis and trans. In the Na-H series the chair conformation was preferred for the trans-isomer 3a while the cis-isomer 3b existed. How can I draw for cis -1-ethyl-3-methylcyclohexane the. Substituents prefer equatorial rather than axial positions in order to minimize the steric hindrance of 13-diaxial interactions.
The conformations of the cis and trans-12-cyclohexanediols and certain of their derivatives. As previously discussed the axial methyl group creates 76 kJmol of steric strain due to 13-diaxial interactions. No in the chair confirmations.
H comb is 6 KJmol lower for the trans isomer cis one equatorial one axial 2 x 30 30 90 KJmol trans two equatorial no axial 30 KJmol trans no equatorial two axial 2 2 x 30 120 KJmol. Cyclohexane is a cycloalkane in which a six-membered carbon ring that exists in the chair conformation. Energy difference values experimentally determined between two chair forms of carious groups to calculate the ratio of the two conformations in solution.
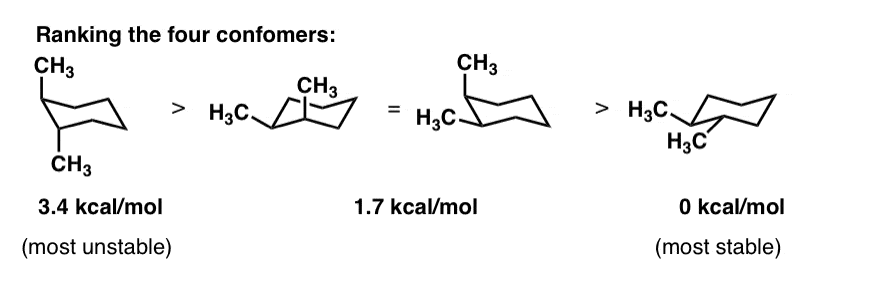
So sis 13 dimethyl cyclo Hexen so could draw it. Lets start by drawing CIS and Trans 13 dimethyl cyclo hexane in their most able chair confirmations. These were both assists.
So this one would be the most stable Azaz. View 72_ Chair Conformations_ Cis and Tans stereoisomerspdf from CHEM 2420 at Tulane University. Cis Trans Chair Conformation.
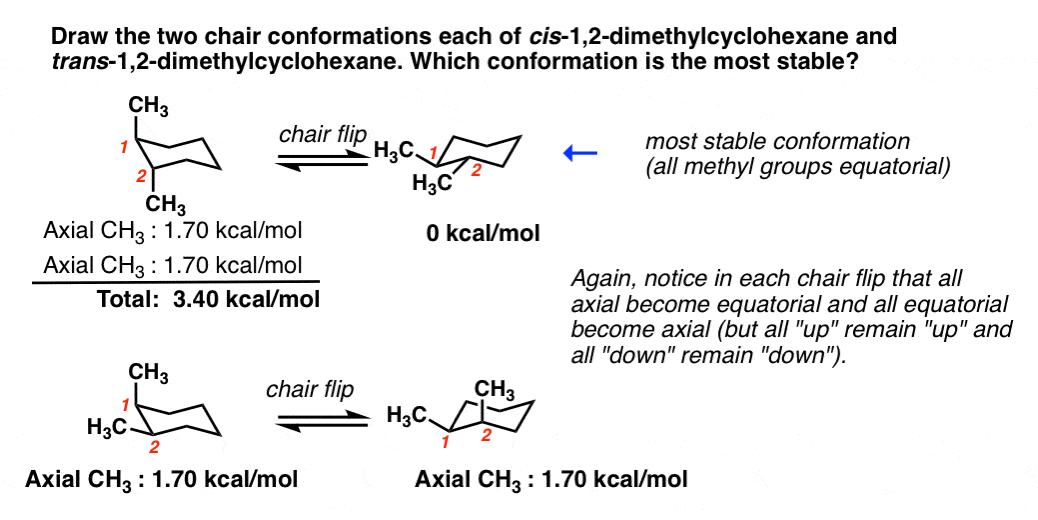
This would be one chair confirmation the second chair confirmation. In cis-12-dimethylcyclohexane both chair conformations have one methyl group equatorial and one methyl group axial. The stable conformations of both the trans- and cis-13-disubstituted Nb-benzyl stereoisomers of the Pictet - Spengler reaction have been determined by NMR spectroscopy and X-ray crystallography in order to better understand the C1 -N2 cis- to trans-isomerization process.
Draw both chair conformations for each of the following isomers. Which is more stable cis- or trans- isomers. What I mean by face is just top or bottom.
Cis and trans stereoisomers of 12-dimethylcyclohexane. Cis and trans is actually going to be based on whether the groups are facing the same face of the ring. Professor Davis explains the cis and trans relationships between positions on cyclohexane molecules.
Cis Trans Chair Conformation. A Indicate by a label whether each methyl group is axial or equatorial. C cis- 13 -dimethylcyclohexane.
C For which isomers is one chair conformation more stable than. It is important to note that both chair conformations also have an additional 38 kJmol of steric strain created by a gauche interaction between the two methyl groups. An important lesson from this exercise is to realize that we cannot propose a good conformation based simply on what we have learned so far.
Recall that cis and trans isomers are diastereoisomers they are not different conformations of the same isomer and cannot be readily interconverted by a simple rotational process a bond would have to be brokenIn the cis isomer here one methyl group is equatorial and one axial and ring flipping simply gives another equivalent conformation. What that means is that Im not going to be looking at positions. H comb is 7 KJmol lower for the cis isomer 12-dimethylcyclohexane.
B For which isomers are the alternative chair conformations of equal stability. Which of these isomers always have equal numbers of axial and equatorial substituents. In the chair conformation this compound has the lowest angle.
If you discover that youre running out of drawing ideas its strongly suggested that you take a walk or simply sit outside for some moment. Both conformers have one methyl group. The key difference between cis cyclohexane and trans cyclohexane is that cis cyclohexane has its substituents pointing to the same plane of the ring whereas trans cyclohexane has its substituents pointing to opposite planes.
A lot of students say Oh but theyre both equatorial so that means that they should be cis. Which exist as equilibrium mixtures of diaxial and. 2 methylcyclohexanol is an alcohol.
Or we could draw like this. Chair conformation of cyclohexane 2. Draw the chairs for cis 2 methylcyclohexanol and trans 2 methylcyclohexanol in the conformation that will undergo e2 elimination.
See whether you can get your idea graphically displayed on a single part of paper. Or one is equatorial and one is axial so they should be trans Wrong. Since one methyl is axial this costs 18 kcal.
The iodine substituent position 1 and methyl group position 3 are cis to each other and should go in the equatorial positions to generate a stable chair conformation. B trans- 12 -dimethylcyclohexane. Chair conformations Cis and Trans Post by JavierMelgoza2E Sun Mar 19 2017 120 am I was wondering whether Cis was with both being the same orientation or the same direction.
They react with oxoacids and carboxylic acids to form esters plus water. Chair conformations are generally more stable than other possibilities so thats all well consider. In todays video I am going to be going over cis-trans stereiosiomers.
A particularly important case comes up with. He comments on how differences in the positions of subs. Overall both chair.
Draw cis and trans 2 methylcyclohexanol in their most stable chair conformations. D trans- 13 -dimethylcyclohexane. Chemistry Organic Chemistry Draw the alternative chair conformations for the cis and trans isomers of 12-dimethylcyclohexane 13-dimethylcyclohexane and 14-dimethylcyclohexane.
Next time youve got an outstanding concept do not. 11-dimethylcyclohexane does not have cis or trans isomers because both methyl groups are on the same ring carbon. Comb is 7 KJmol lower for the trans isomer 13-dimethylcyclohexane.
Cyclohexane Conformational Analysis
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts
Possible Chair Conformations Of 1 2 Dimethylcyclohexane

For Cis 1 3 Dimethylcyclohexane Which Two Clutch Prep
Cyclohexane Conformational Analysis

Cyclohexane Chair Conformation Stability Which One Is Lower Energy
Cyclohexane Chair Conformation Stability Which One Is Lower Energy
Cyclohexane Chair Conformation Stability Which One Is Lower Energy
Stereoisomers Of Cyclic Compounds Chemgapedia
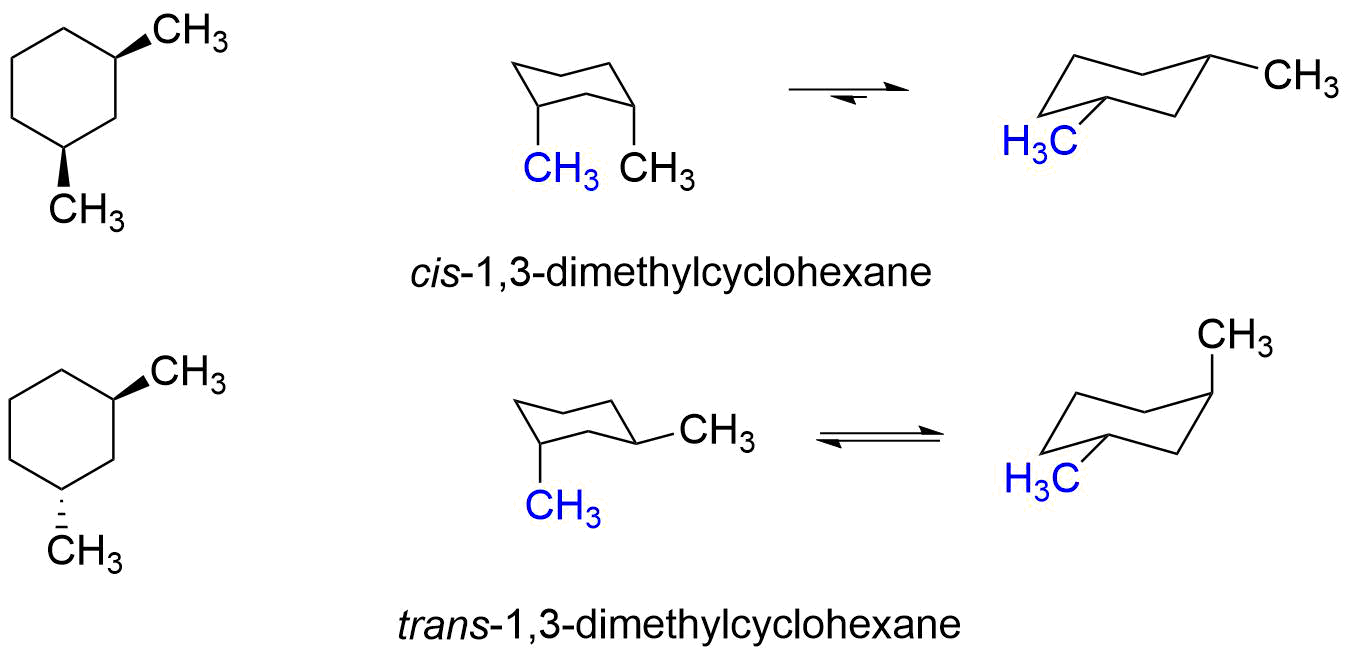
3 9 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts
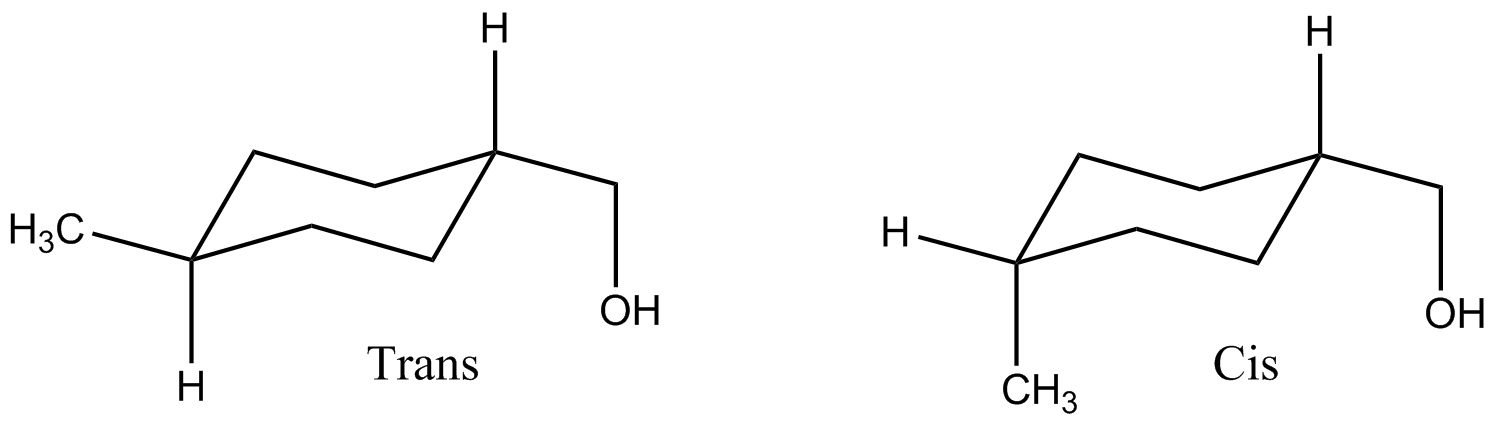
File Cis And Trans 1 Methyl 4 Hydroxymethyl Cyclohexane Png Wikipedia
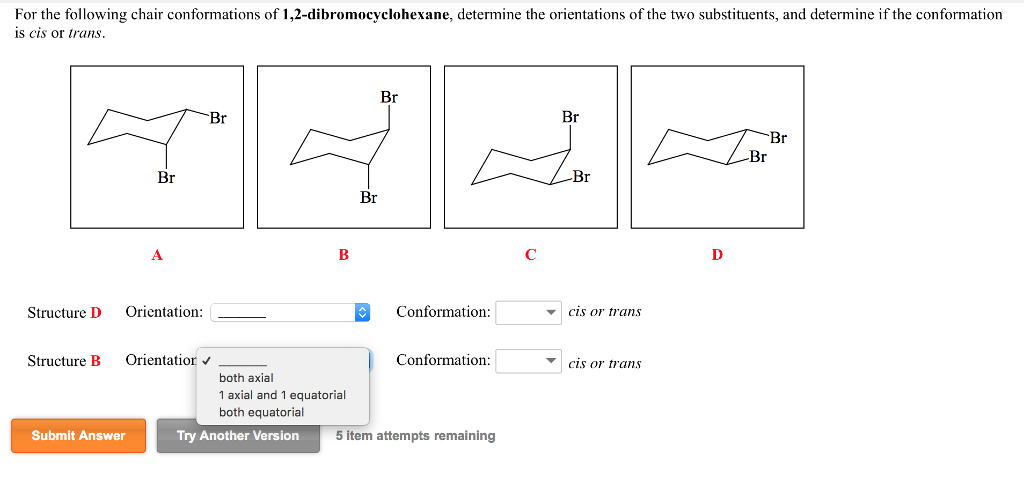
For The Following Chair Conformations Of Chegg Com
Cyclohexane Conformational Analysis
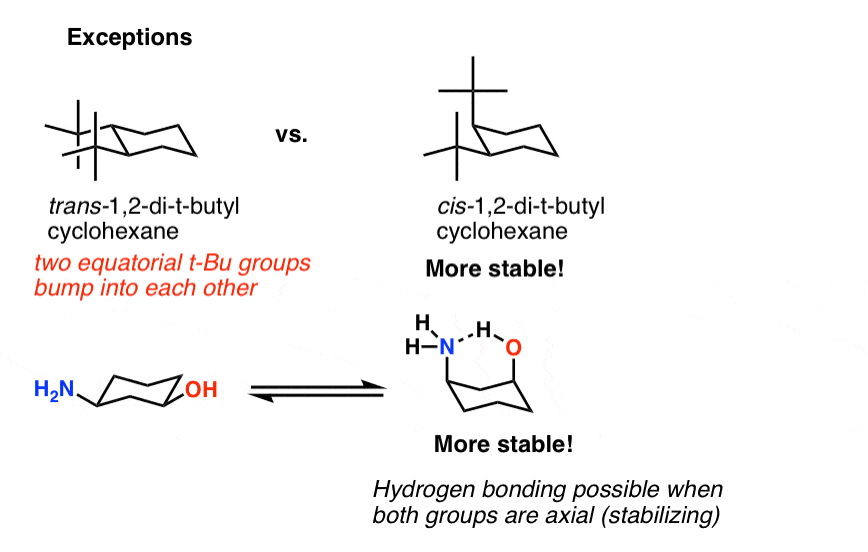
Cyclohexane Chair Conformation Stability Which One Is Lower Energy
4 10 Conformations Of Disubstituted Cyclohexanes Chemistry Libretexts

Which One Of The Following Chair Conformations Will Be More Stable Explain Your Answer By Drawing Conformational A Cis 1 T Butyl 4 Methylcyclohexane B Trans 1 T Butyl 4 Methylcyclohexane Study Com

Organic Chemistry Stereoisomerism Of Chair Conformation Youtube